What Kind of Crystalline Solid Is Copper Apex
What kind of crystalline solid is copper what is ductility in a solid what is an amorphous solid apex what are crystalline solids apex. Graphite is a covalent-network type of crystalline solid.
2 Show answers Another question on Chemistry.

. Ne Ar Kr Xe Rn Quite soft. Poor electrical and thermal conductors Tm increases with increasing molar mass. Amorphous Solids They are not rigid so mild effects may change the shape.
Crystalline solids consist of atoms ions and molecules arranged in definite and repeating three-dimensional patterns. Inorganic salts like sodium chloride magnesium sulphate potassium bromide copper sulphate caesium chloride etc. Crystalline solids are those solids that have a sharp melting point because they have definite heat of fusion and amorphous solids are those solids that do not have a sharp melting point because they do not have definite heat of fusion.
10 Is diamond a non conductor. 12 What is the appearance of diamond. 6 Is diamond ionic or covalent.
There are four types of crystalline solids. Amorphous Solids Isotropic in nature. Copper Crystal Structure.
Crystalline Solids They are rigid solids and applying mild forces will not distort its shape. What kind of crystalline solid is copper. Are all crystalline solids.
Three types of Atomic solids. In metals and in many other solids the atoms are arranged in regular arrays called crystals. There are two main categories of solids.
Metallic copper Network Covalent diamond Noble gases. Properties of Crystalline Solids. The properties of crystalline solids are.
The atoms ions and molecules in a crystalline solid are arranged in such a way that they have a definite shape and structure known as characteristic geometry. Crystalline solids are those in which the atoms ions or molecules that make up the solid exist in a regular well-defined arrangement. Metallic solids like gold.
Metallic Bond Excellent conductor. Non-metallic solids like sulphur phosphorus iodine are Crystalline Solids. 13 What are.
Admin Send an email December 15 2021. 5 Why type of solid is diamond. Covalent bond Hard and high melting point.
3 Does diamond form a molecular solid. A possible crystal structure of Copper is face-centered cubic structure. A few examples of crystalline solids include sodium chloride quartz diamond etc.
They consist of a positively charged cation and a negatively charged anion. 1 Types of Crystalline Solids Atomic solids consist of atoms The atoms are held together by weak dispersion forces Examples. 9 What kind of solid is diamond C apex.
Solids that have a regular and three-dimensional arrangement of constituent particles such as atoms molecules or ions are known as crystalline solids. Ionic solids Made up of positive and negative ions and held. Unlike amorphous solids that melt at a range of temperatures crystalline solids have definite melting points.
11 Is graphite a solid. In the following lines we shall compare the properties of crystalline and amorphous solids. A crystal lattice is a repeating pattern of mathematical points that extends throughout space.
Copper is a crystalline solid. Who invented the word cell. Most of the solids are crystalline in structure.
4 Is diamond is a covalent solid. See more articles in category. The forces of chemical bonding causes this repetition.
Ie the magnitude of the physical properties is the same along with all directions of the solid. Crystalline solids include metallic ionic network atomic and molecular solids and true solids are crystalline. When Ionic Solids dissolve in water the cations and the anions separate and become free to move about in water thereby allowing the solution to conduct electrical current.
Force and property of Mettalic solid. Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday. Platinum silver copper zinc etc.
Force and properties of network covalent solid. 8 Which is a covalent solid. Lattice points made of atoms.
7 Is C diamond a metallic solid. Ionic Solids are solids which are composed of oppositely charged ions such as KCl.
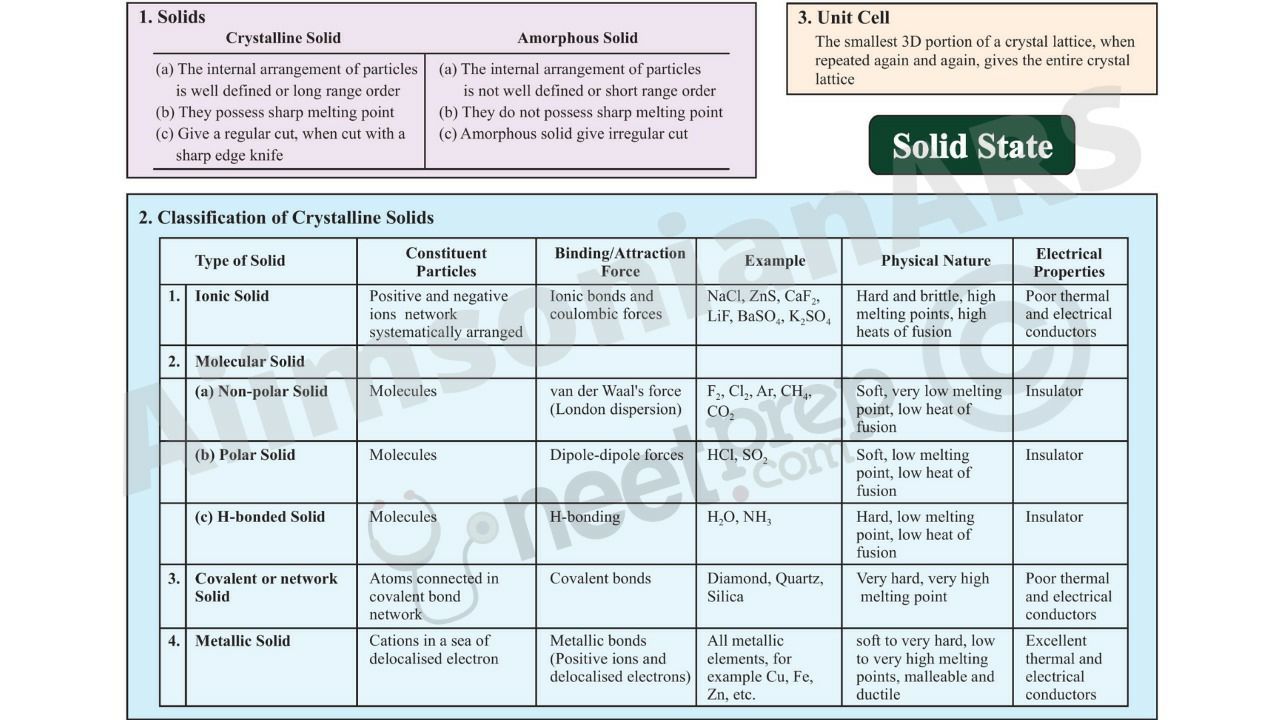
Ncert Ebook For The Solid State The Solid State Chapter 1 Ncert Chemistry Xii

Solid State Archives Page 2 Of 2 The Fact Factor
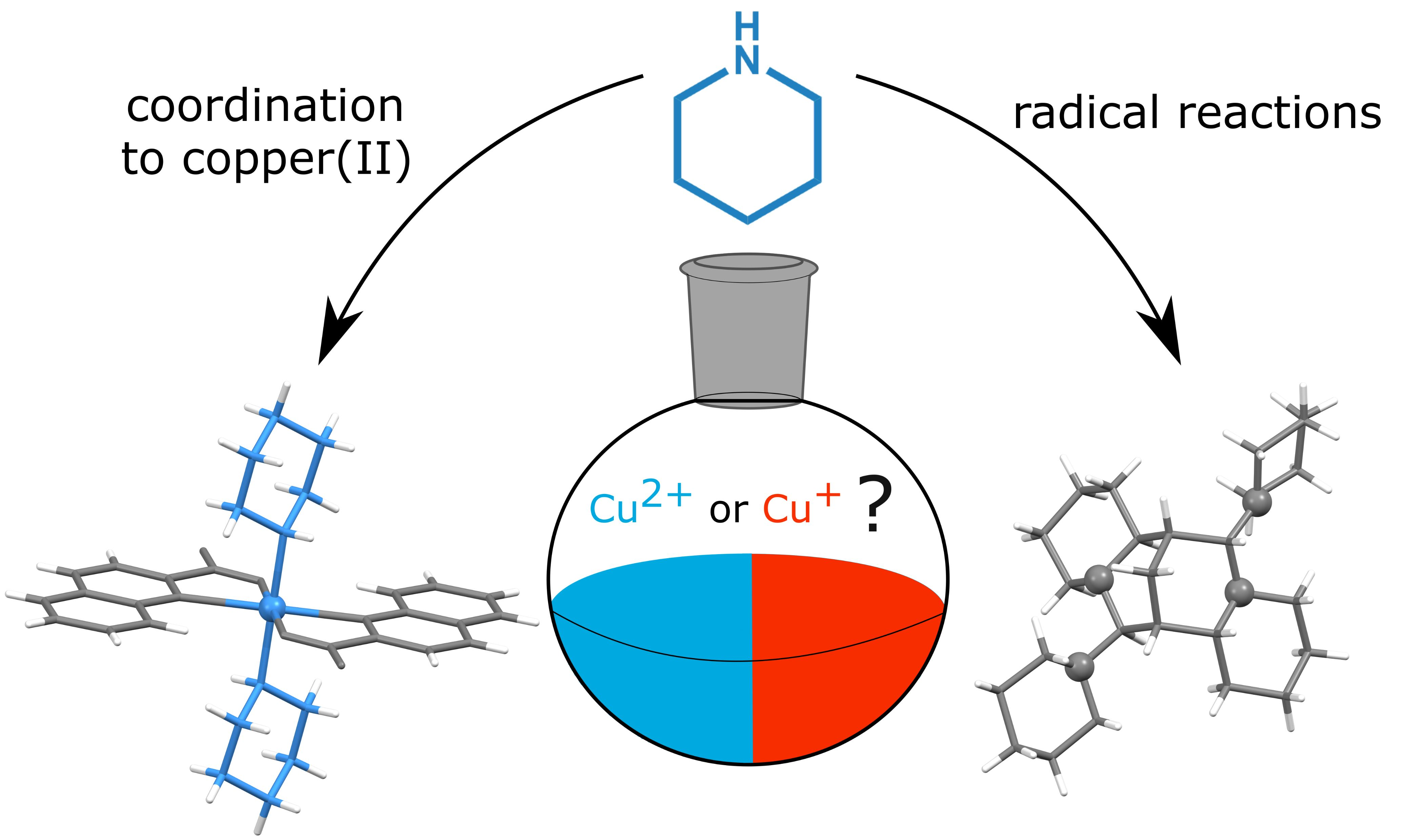
Molecules Free Full Text Beyond The Simple Copper Ii Coordination Chemistry With Quinaldinate And Secondary Amines Html
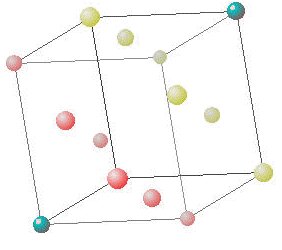
Solids First Year General Chemistry

Crystalline And Amorphous Solids Explanation Differences Examples Etc
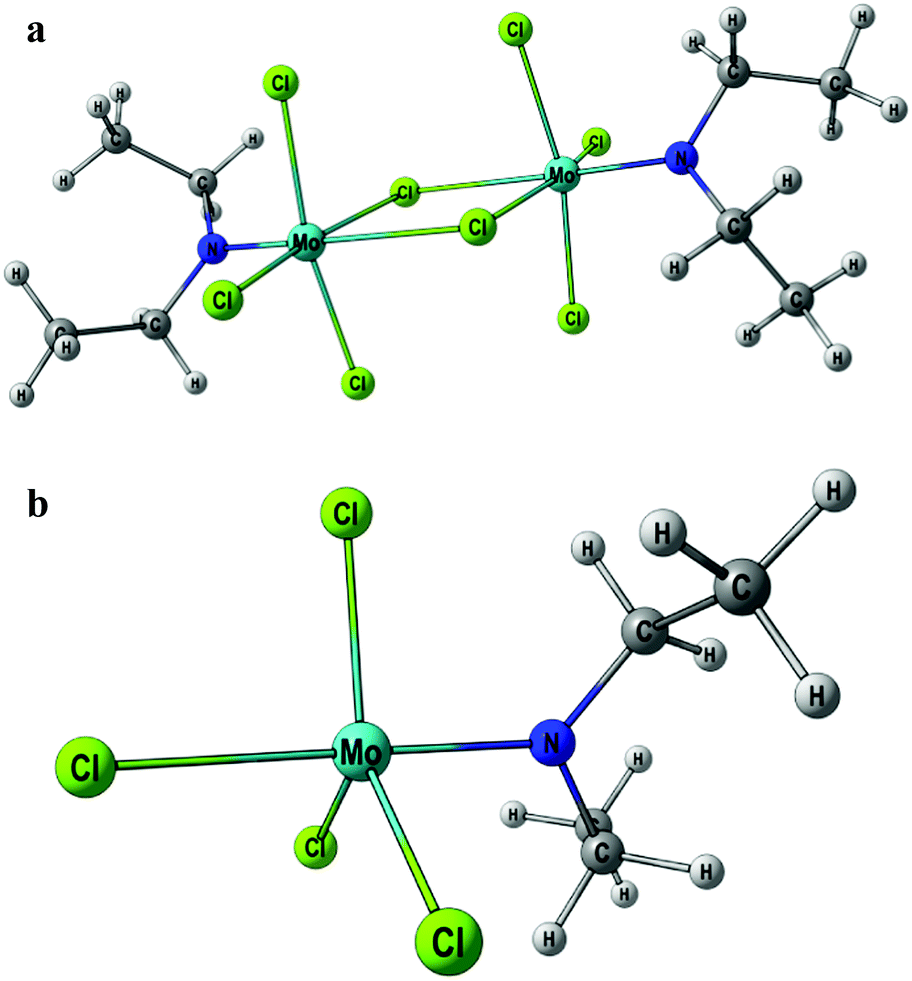
Unusual Activation Pathways Of Amines In The Reactions With Molybdenum Pentachloride New Journal Of Chemistry Rsc Publishing Doi 10 1039 C6nj03992h

Synthesis And Hydrogen Bond Patterns Of Aryl Group Substituted Silanediols And Triols From Alkoxy And Chlorosilanes Kannengiesser Chemistry A European Journal Wiley Online Library

Pdf Origins Of Contrasting Copper Coordination Geometries In Crystalline Copper Sulfate Pentahydrate

To Perform The Process Of Crystallization Of Copper Sulphate Neb Class 7

Crystalline And Amorphous Solids Explanation Differences Examples Etc
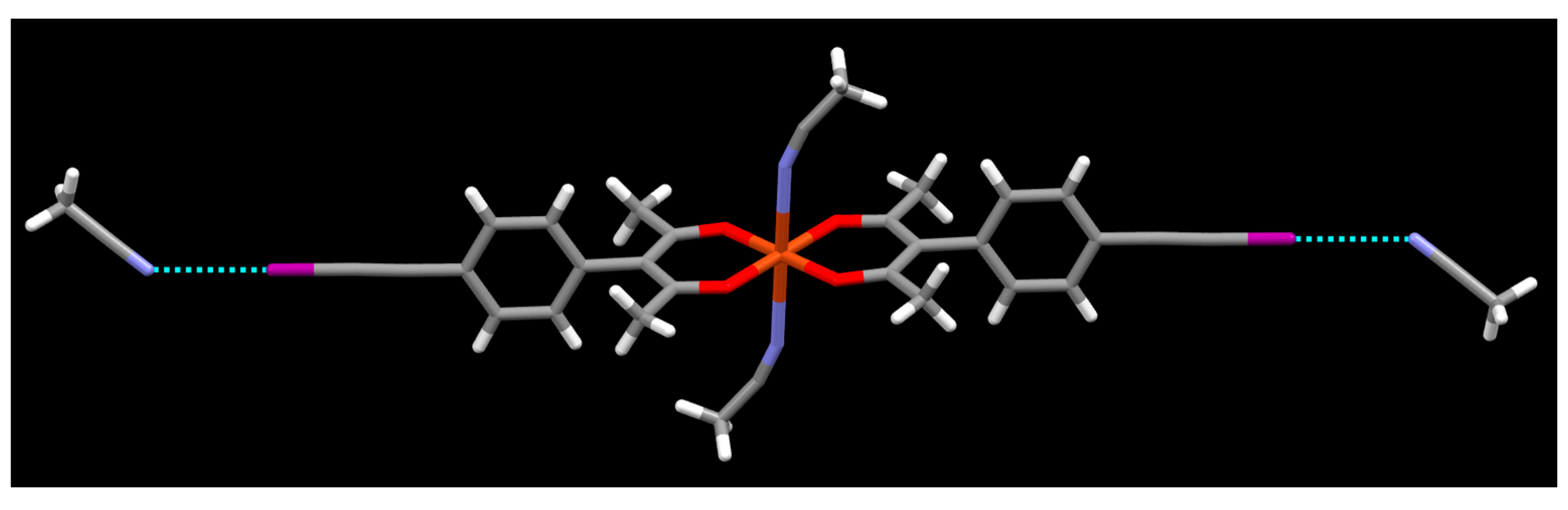
Crystals Free Full Text The Role Of Halogen Bonding In Controlling Assembly And Organization Of Cu Ii Acac Based Coordination Complexes Html
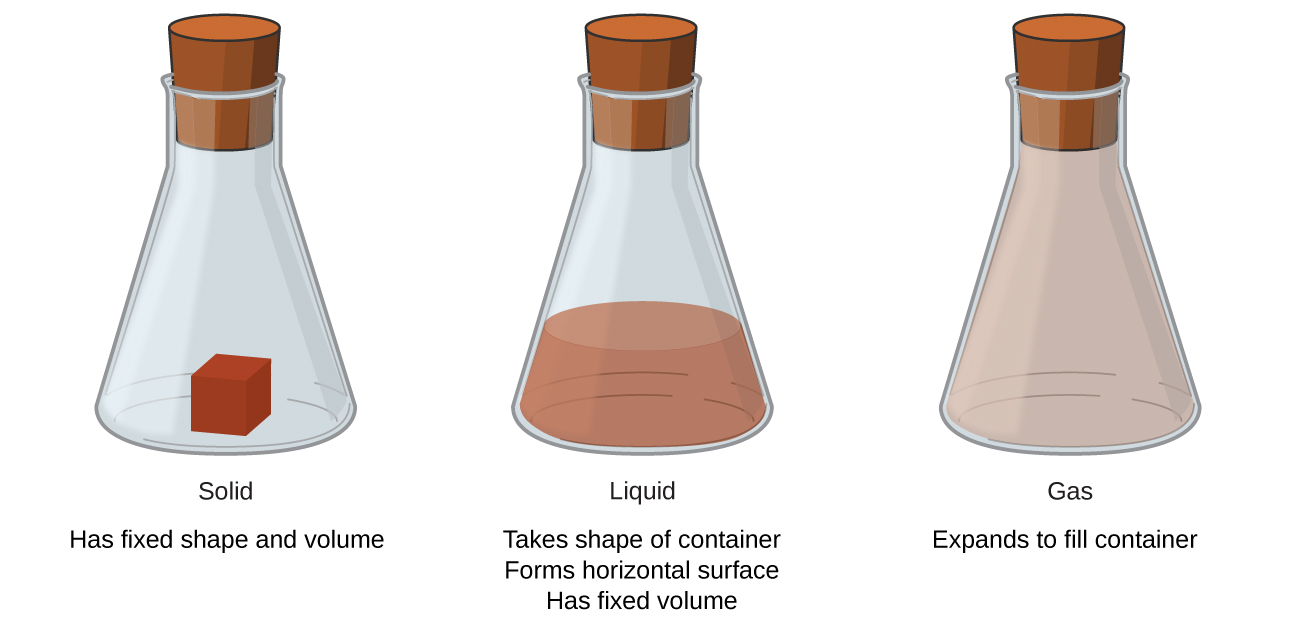
1 2 Phases And Classification Of Matter Chemistry
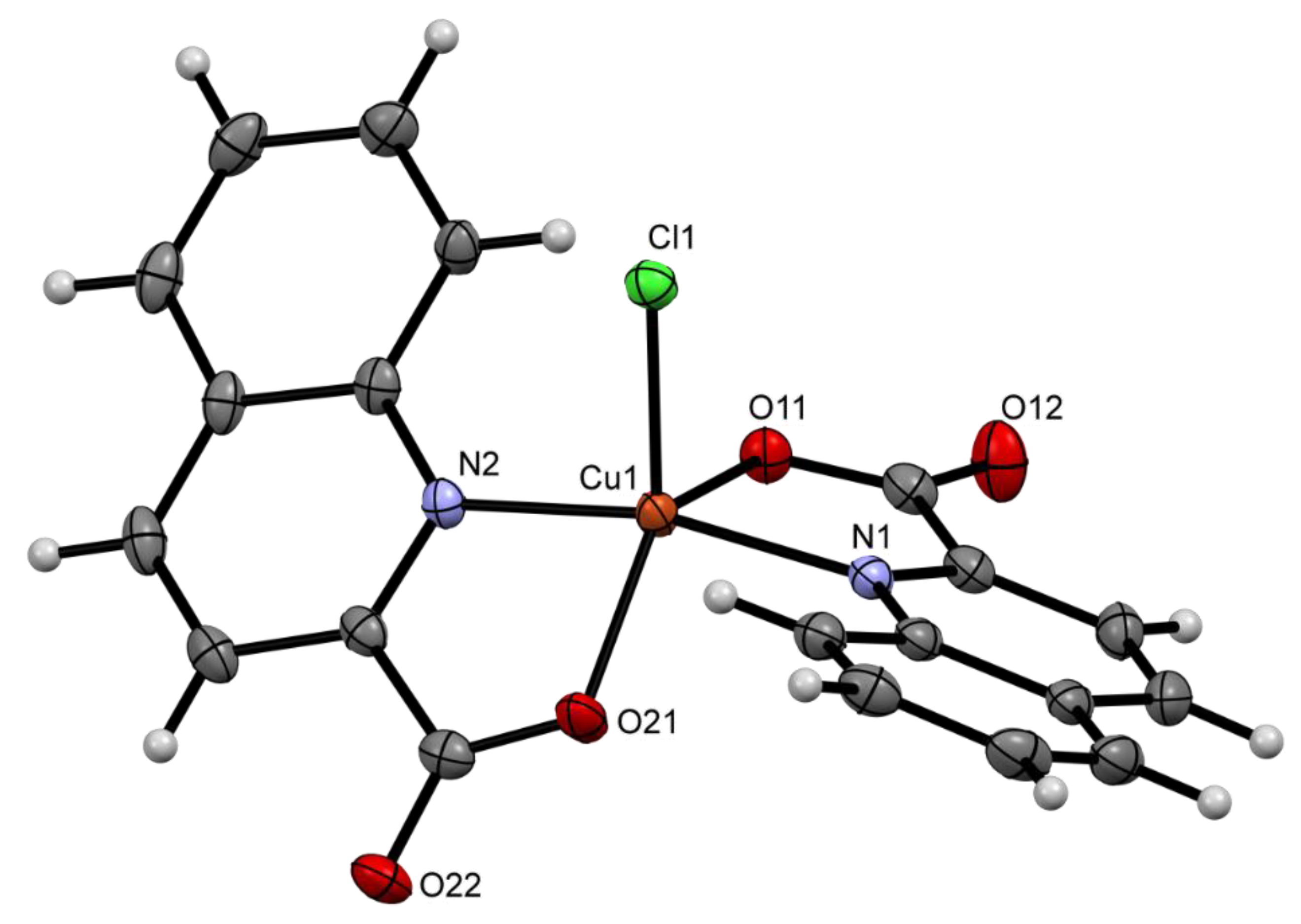
Molecules Free Full Text Beyond The Simple Copper Ii Coordination Chemistry With Quinaldinate And Secondary Amines Html

Crystal Types Of Bonds Britannica

Pdf Origins Of Contrasting Copper Coordination Geometries In Crystalline Copper Sulfate Pentahydrate
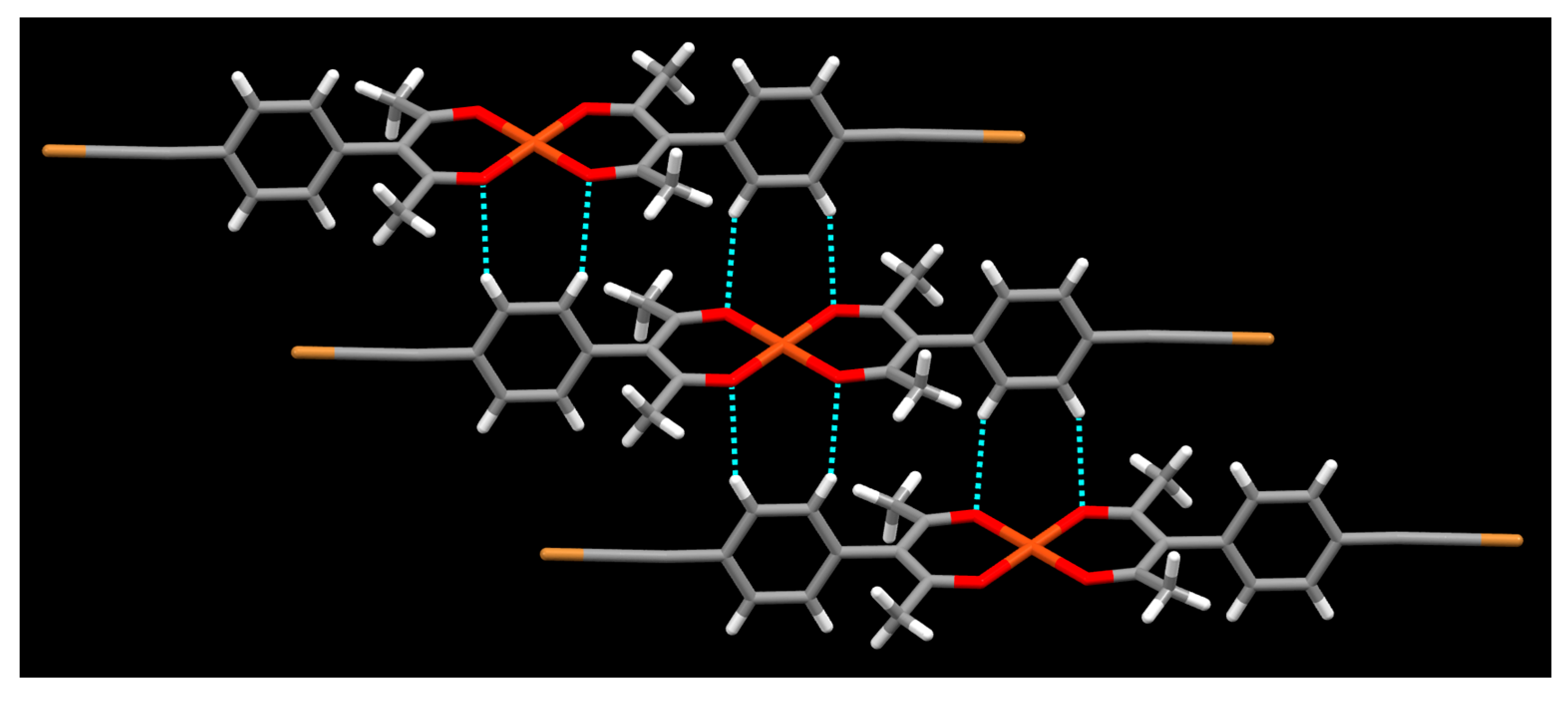
Crystals Free Full Text The Role Of Halogen Bonding In Controlling Assembly And Organization Of Cu Ii Acac Based Coordination Complexes Html
Molecules Free Full Text Beyond The Simple Copper Ii Coordination Chemistry With Quinaldinate And Secondary Amines Html
Comments
Post a Comment